Introduction
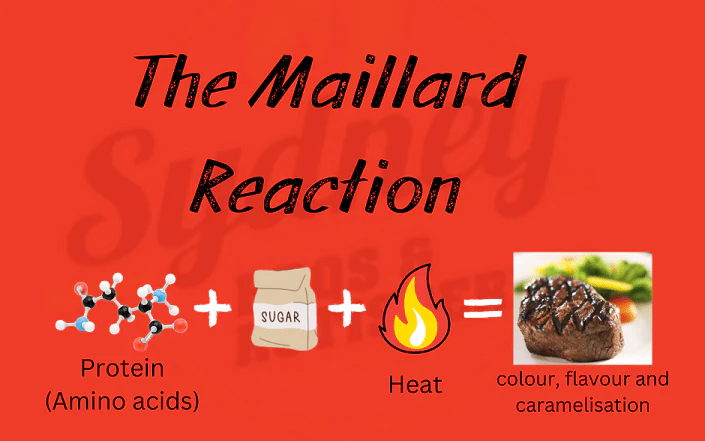
In the realm of culinary chemistry, few reactions hold as much significance as the Maillard reaction. Named after French chemist Louis-Camille Maillard, this complex chemical process occurs when heat is applied to amino acids and reducing sugars, leading to a cascade of transformations that imbue foods with irresistible flavors, enticing aromas, and visually appealing hues.
Understanding the Maillard Reaction
Chemical Mechanism
The Maillard reaction initiates when amino acids, the building blocks of proteins, react with reducing sugars in the presence of heat, typically above 285°F (140°C). This reaction proceeds through a series of intricate steps, including sugar fragmentation, dehydration, and the formation of numerous flavorful compounds, such as furans, pyrazines, and thiols.
Browning Process

A hallmark of the Maillard reaction is the browning effect it imparts on foods, transforming their appearance from pale to golden brown. This caramelization of surface sugars not only enhances visual appeal but also contributes to the development of complex flavors and textures, elevating the culinary experience.
Factors Influencing the Maillard Reaction
Temperature
The rate and extent of the Maillard reaction are highly dependent on temperature. Higher temperatures accelerate the reaction, leading to more rapid browning and flavor development. However, excessively high temperatures can also result in burnt flavors and undesirable bitterness, necessitating careful temperature control.
pH Level
The pH level of the cooking environment significantly influences the Maillard reaction. While the reaction occurs optimally in slightly alkaline conditions, variations in pH can alter the rate and intensity of browning and flavor formation. Acidic ingredients, such as vinegar or citrus juice, may inhibit the Maillard reaction, whereas alkaline substances, like baking soda, can enhance its progression.
Moisture Content
The presence of moisture plays a crucial role in the Maillard reaction. While a minimal amount of moisture is necessary to facilitate chemical reactions, excessive moisture can inhibit browning and instead promote steam-related cooking processes, such as boiling or steaming. Consequently, achieving the ideal balance of moisture is essential for optimizing the Maillard reaction.
Culinary Applications
Flavor Enhancement

The Maillard reaction is instrumental in enhancing the flavor complexity of various foods, ranging from meats and vegetables to baked goods and roasted coffee beans. By carefully controlling cooking conditions and ingredient composition, chefs can harness the transformative power of the Maillard reaction to elevate the taste profiles of their creations.
Texture Development
In addition to flavor enhancement, the Maillard reaction also contributes to the development of desirable textures in cooked foods. The formation of caramelized crusts on grilled meats, crispy edges on baked goods, and golden sears on pan-fried vegetables are all outcomes of the Maillard reaction, enhancing both visual appeal and mouthfeel.
Conclusion
The Maillard reaction stands as a cornerstone of culinary science, driving the formation of flavors, aromas, and textures that define the art of cooking. By understanding the underlying chemistry and factors influencing this reaction, chefs and home cooks alike can unlock a world of culinary possibilities, infusing their creations with depth, complexity, and visual allure. Embrace the transformative power of the Maillard reaction and embark on a journey of culinary exploration and innovation.